This information HAS errors and is made available WITHOUT ANY WARRANTY OF ANY KIND and without even the implied warranty of MERCHANTABILITY or FITNESS FOR A PARTICULAR PURPOSE. It is not permissible to be read by anyone who has ever met a lawyer or attorney. Use is confined to Engineers with more than 370 course hours of engineering.
If you see an error contact:
+1(785) 841 3089
inform@xtronics.com
Try to use only 12V lead acid cells for your applications. They are the most popular type and are least likely to have been on the shelf for too long. When you buy your batteries, check their voltages. If the battery terminal voltage is not above 12 volts, send the batteries back!
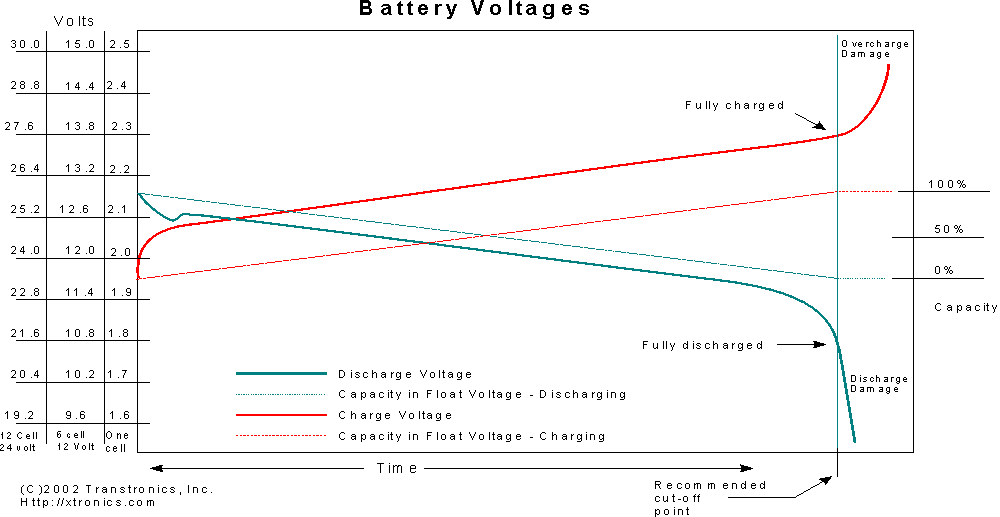
There is much confusion about "battery voltage" because a battery has more than one voltage and often the literature is lax in defining which voltage is being discussed at the time. Also, measurements of a batteries voltage, particularly float voltage, require time (that is often not allotted or specified) for the battery to stabilize.
Charge voltage is usually stated at the battery capacity in Ah divided by 5 (Ah/5). Thus, a 10Ah battery would use a 2A charge rate to specify the battery Charge Voltage. This voltage varies with the charge state of the battery, that is it is higher when the battery has a full charge.
In a similar manner Discharge voltage is usually specified at a current that is capacity in Ah divided by 20. A 10Ah battery would have a load of 0.5A on it while measuring this voltage.
| Number of Cells |
Nominal Voltage |
Fully-Charged Float Voltage |
Fully-Discharged Float Voltage |
Discharge Voltage at Ah/20 |
Charge Voltage at Ah/5 |
|---|---|---|---|---|---|
| 1 | 2 | 2.15 | 1.9 | 2.0 - 1.7 | 2.1 - 2.30 |
| 6 | 12 | 12.9 | 11.4 | 12 - 10.2 | 12.6 - 13.8 |
| 12 | 24 | 25.8 | 22.8 | 24 - 20.4 | 25.2 - 27.6 |
Energy efficiency is calculated on the amount of power used from the battery while discharging divided by the amount of power delivered to the battery while charging, multiplied by 100 to yield percent. Pout x 100 /Pin . A lead-acid battery has an efficiency of only 75-85%. (this includes both the charging loss and the discharging loss. From zero State of Charge(SOC) to 84% SOC the average overall battery charging efficiency is 91% ( A Study of Lead-Acid Battery Efficiency Near Top-of-Charge and the Impact on PV System Design ) the balance is losses during discharge. The energy lost appears as heat and warms the battery. Keeping the charge and discharge rate of a battery low, helps keep a battery cool and improves the battery life. We are ignoring the self discharge losses.
The above losses don't include losses in the charging circuit which may have an efficiency of anywhere from 60% to 80% - thus the overall- total efficiency is the product of these efficiencies and ends up being 45 to 68%. (To further this example and to show why physics and not some corporate conspiracy is the reason we don't have electric cars - suppose the controls and motors on a car were 85% - the over all efficiency is now only 38 - 58%. You can see that an electric car would use about twice the energy than a conventional car - not to mention the great cost of the regular replacement of batteries. This is why batteries are best used where only intermittent, or very low power use is required.)
To further explain - If the electricity is generated from a gasoline engine - and that energy is converted to electricity, and then sent through power line transformers and power lines, and then converted to DC, and then converted to chemical energy, and then converted back to electrical energy, and then converted to rotary mechanical energy - it is clear that many losses have occurred. If the same gasoline motor was providing the rotary energy directly to the drive train, it is much more efficient.
Battery capacity refers to the total amount of energy stored within a battery. Rated capacity is in Ampere-hours (AH), which is the product of the current times the number of hours to total discharge. The capacity is normally compared with a time of 20 hours and a temperature of 68°F (20°C). There are five factors that dictate the capacity of a given battery:
Specifying battery capacity involves a bit more than multiplying the load current by the backup time in hours. You must first de-rate the battery for capacity tolerance, temperature, and discharge rate.
Example -- 10 Hours @ 200 mA, average current, worst case temp is 0°C
Total 3.7 AH
Discharging a battery even slightly below its fully discharged voltage shortens its life. Letting a battery sit and self discharge to 0 destroys the battery.
Also, consider that some equipment does not stop working "gracefully" as its voltage supply gets lower and lower. Some electronic equipment may work erratically and cause outputs to turn on and off randomly.
Store batteries at a low temperature if possible, 5°C is ideal. Although capacity goes up with temperature, the life of a battery goes down.
The self discharge rate goes down with temperature. At room temperature, recharge stored batteries every 6 months; storage at 5°C lets you wait 18 months before recharging.
Pick a power supply that can provide for all of your maximum load. Using the battery to pick up the load difference between a small supply and a large, but intermittent load, will keep the battery constantly charging and will ruin the battery in a short time. It is much less expensive to buy a larger power supply than to replace batteries repeatedly.
Taper charging is the default charging mechanism of the BVUPS. Adjust your power supply to 13.8 VDC (27.6 VDC for the 24 volt model). Your battery will charge rapidly at first and then slow down as it reaches full charge. After charging the battery fully, you should see a charge current that is equal to the capacity in Amp hours divided by 100 to 200.
Using an 8 AH Battery, 8/200 = .040A, to 8/100 = .080A; therefore the final charge current should be between 40mA and 80mA.
The advantage to a Constant Voltage system is simplicity. On the other
hand, it is a slow way to recharge a battery. Increasing the voltage to
speed up the charge rate will cause the battery to overcharge and fail.
Instead, speed up battery recharge with a constant current charge
system.
This is the preferred method of charging Lead acid low maintenance batteries. Charge current is limited to a maximum Current AND voltage. The table below is based on I = Ah/4
| Nominal Capacity Ah | 1.2 | 1.9 | 2.6 | 4 | 7 | 17 | 21 | 33 |
|---|---|---|---|---|---|---|---|---|
| Maximum Initial Charge rate A | 0.3 | 0.475 | 0.65 | 1 | 1.75 | 4.25 | 5.25 | 8.25 |
| No load - fully charged voltage | 2.03V/cell |
|---|---|
| Charging over-voltage | 0.3V/cell |
| Discharge drop-voltage | 0.05V/cell |
| Full charge with float current | 2.3V/cell |
| Fully discharged Battery | 1.6 - 1.8V /cell |
| dE/dT Fully charged battery | |
| dE/dT full discharged Battery | -0.00043 V/C per cell |
The ones available in SPICE simulators are just a bunch of capacitance and with resistance in between which is quite different from a real battery. A real 12V battery will show 13.8V on a trickle charge and drops to 12V with just the slightest load and with a practical load it drops another .05V per cell or about 0.3 volt to put a 12V battery at 11.7V. Thus, there is a dead band in the battery that has nothing to do with internal resistance.
What is interesting is that the no load voltage stays pretty much the same until the battery is really discharged. The battery also drops farther upon applying a load. The reverse is true for charging a battery. The zero current voltage is always very close to 12V but as the battery reaches full charge the jump in voltage on application of a charging current increases.
I always worried about the Temperature coefficient of lead-acid batteries. dE/dT turns out zero in a fully charged battery. There is a temp-co for a discharged battery but dE/DT = -0.000 43 V/C (per cell) so it can be safely ignored. I think others have confused batteries dE/dT with dI/dT which does have a noticeable temp-co.
A batteries internal resistance is not linear with Ah capacity, but can be assumed linear at low power levels. A graph of it is asymptotic to both the y and x axis in the first quadrant. The slope is pretty linear at the power levels we work with, but much of the literature on batteries came from submarines work where the systems Ah rating is magnitudes larger.
Email(C) Copyright 1994-2019
All trademarks are the property of their respective owners.